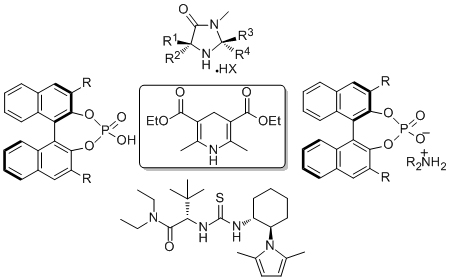
Enzyme mimics
Aldehyde dehydrogenases (ALDH) are NAD(P)-dependant enzymes capable of the selective oxidation/reduction of aldehydes vital in important biological functions such as glycolysis, bacterial aspartate synthesis and drinking beer. We have recently designed the first artificial keto-reductase mimicking system capable of cofactor regeneration and recycling (above) and we now wish to design a fully bifunctional artificial ALDH which we envisage will be active in a variety of transformations for which no catalytic technology is currently available.
'Asymmetric organocatalytic reductions mediated by dihydropyridines'
S. J. Connon*, Org. Biomol. Chem. 2007, 5, 3407.
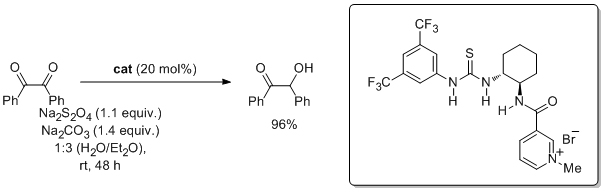
'A reductase-mimicking thiourea organocatalyst incorporating a covalently bound NADH analogue: efficient 1,2-diketone reduction with in-situ prosthetic group generation and recycling'
B. Procuranti and S. J. Connon*, Chem. Commun. 2007, 1421.